OUR RESEARCH
Amyloid aggregation: functional and disease related
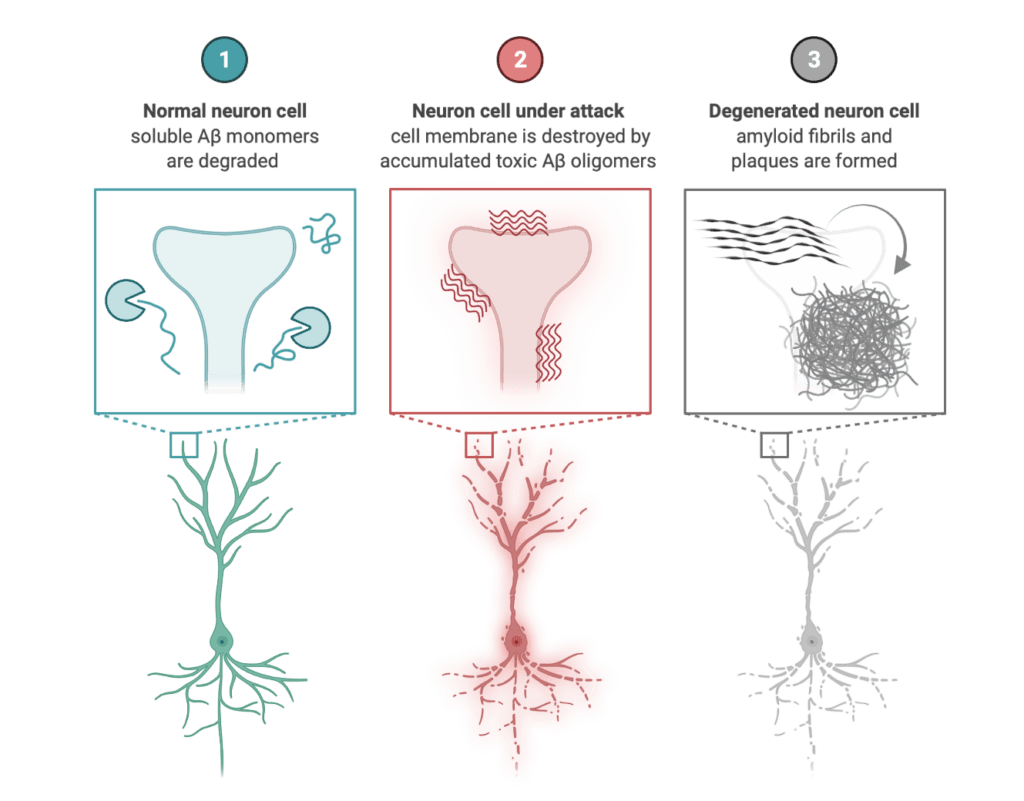
Amyloid protein aggregation is a wide-ranging phenomenon and is of great importance in several areas of science as such aggregates can lead to diseases or functional and contributing to normal biological processes.Unfortunately, the detailed molecular mechanism of the amyloid aggregation is still not fully resolved. To this end, we are employing full-atomistic or coarse-grained molecular dynamics simulations of amyloid-beta peptide (Aβ), human islet amyloid polypeptide (hIAPP), Huntingtin, PI3-Kinase at the presence of neuronal membranes, carbohydrates, molecular crowders and/or at different ionic strengths to gain insight on the possible biophysical mechanisms of amyloid aggregation manifested through the following research project:
- Simulating Aβ-membrane interactions considering in vivo conditions.
- Developing hybrid simulation models to study Aβ42 aggregation.
- Simulating Amyloid aggregation in solution.
- Exploring intrinsically disordered proteins and their propensity to aggregate into amyloid beta structures.
A brief history of amyloid aggregation simulations
H Fatafta, M Khaled, B Kav, OO Olubiyi, B Strodel
Wiley Interdisciplinary Reviews: Computational Molecular Science 14 (1), e1703, 2024
A hairpin motif in the amyloid-β peptide is important for formation of disease-related oligomers
M. Khaled, I. Rönnbäck, LL. Ilag, A. Gräslund, B. Strodel, N. Österlund
Journal of the American Chemical Society 145 (33), 18340-18354, 2023
Transition Networks Unveil Disorder-to-Order Transformations in Aβ Caused by Glycosaminoglycans or Lipids
M. Schäffler, S. Samantray, B. Strodel
International Journal of Molecular Sciences (2023)
Structural dissection of the first events following membrane binding of the islet amyloid polypeptide
L. Khemtemourian, H. Fatafta, B. Davion, S. Lecomte, S. Castano, B. Strodel
Frontiers in molecular biosciences 9, 849979, (2022)
Amyloid-β peptide dimers undergo a random coil to β-sheet transition in the aqueous phase but not at the neuronal membrane
Fatafta H, Khaled M, Sayyed-Ahmad A, Strodel B
Proceedings of the National Academy of Sciences (2021)
Disorder-to-order transition of the Amyloid-beta peptide upon lipid binding
H Fatafta, B Kav, BF Bundschuh, J Loschwitz, B Strodel
Biophysical Chemistry, Volume 280, Pages 106700 (2021)
Different force fields give rise to different amyloid aggregation pathways in molecular dynamics simulations
S Samantray, F Yin, B Kav, B Strodel
Journal of Chemical Information and Modeling, Vol. 60, Pages 6462-6475 (2020)
This project is funded by
- Forschungszentrum Jülich
- Palestinian-German Science Bridge
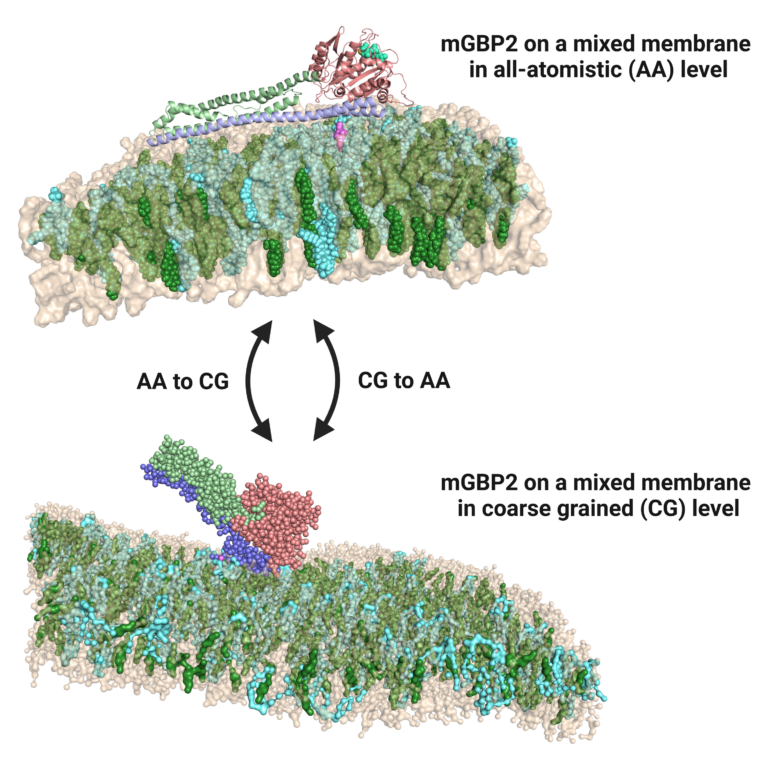
Infection biology and membrane simulation
Pathogen-host interactions provoke the immune reaction of γ-interferons, which in turn regulate a multitude of proteins. Upon Toxoplasma gondii infection, murine guanylate binding proteins (mGBPs) prove vital for mice survival.
Here, mGBPs localize to the parasitophorous vacoule membrane of the parasite. In order to elucidate their mechanism of action, we perform atomistic and coarse-grained MD simulations, generating results about binding mode, lipid
specificity and possible membrane disruption. Furthermore, we study the dynamics of a dynamin-like hinge motion using enhanced sampling methods and possible dimer structures using docking. Close collaboration with experimentators from CRC1208 (HHU, Klaus Pfeffers group, Institute of Medical Microbiology and Hospital Hygiene) allows continuous cross-verification of our joined findings.
Domain motions, dimerization, and membrane interactions of the murine guanylate binding protein 2
J. Loschwitz, N. Steffens, X. Wang, M. Schäffler, K. Pfeffer, D. Degrandi, B. Strodel
Scientific Reports 13 (1), 1-20 (2023)
Biochemical and structural characterization of murine GBP7, a guanylate binding protein with an elongated C-terminal tail
L. Legewie, J. Loschwitz, N. Steffens, M. Prescher, X. Wang, S.H.J. Smits, L. Schmitt, B. Strodel, D. Degrandi, K. Pfeffer
Biochem. J. 476, 3161-3182 (2019)
Large-scale, dynamin-like motions of the human guanylate binding protein 1 revealed by multi-resolution simulations
B. Barz, J. Loschwitz, B. Strodel
PLOS Comp. Biol., 15, e1007193 (2019)
- Collaborative Research Center 1208
- Leibniz-Rechenzentrum
- Jürgen Manchot Stiftung
Computational enzyme design
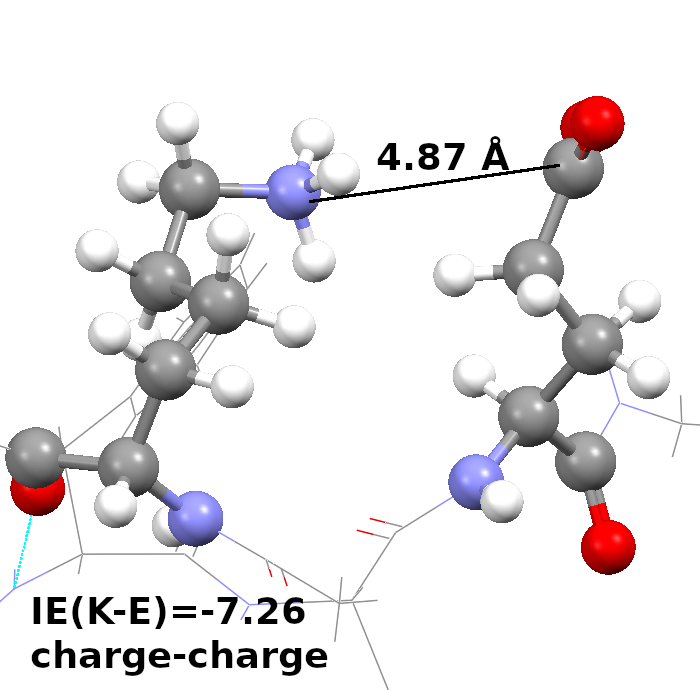
Enzymatic biocatalysis constitutes a promising environmentally friendly alternative to harmful industrial processes, but the stability, activity and selectivity/specificity is most often not sufficient for biotechnological application. In collaboration with experimental groups, as well as experts in highly contemporary computational methods, we address the engineering of organic solvent tolerant esterases and poly(ethylene) terephthalate (PET) degrading enzymes using all-atom standard and accelerated molecular dynamics (MD) as well as hybrid quantum mechanics/molecular mechanics (QM/MM) simulations complemented by state-of-the-art machine learning for structure-activity relationship predictions.

Lara Scharbert
Show DetailsThis project is funded by Deutsche Bundesstiftung Umwelt.
Computer-aided drug design
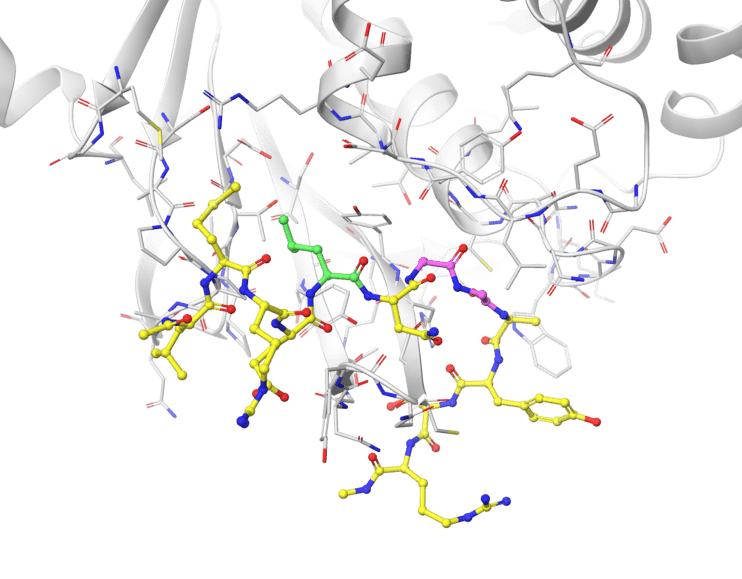
When COVID-19 became a global pandemic in the spring of 2020, we initiated the computer-aided drug design (CADD) project to contribute to the discovery and development of a prospective drug against SARS-CoV-2.
We screened over one million compounds by docking them against the main protease (3CLpro) and used molecular dynamics (MD) simulations to identify compounds with the highest binding affinity.
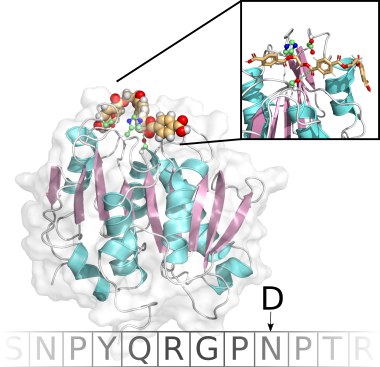
Our current work is focused on peptide ligands or peptidomimetic agents against various diseases, including D-peptides as protease-inhibitors against SARS-CoV-2 or radiopharmaceuticals as tumor-markers.
Together with our collaborators at the Forschungszentrum Jülich and the Heinrich-Heine University we are able to investigate the ligands holistically for their drug or theranostic potential through in silico, in vitro and in cell approaches.
The Theranostic Optimization of PSMA-GCK01 Does Not Compromise the Imaging Characteristics of [99mTc]Tc-PSMA-GCK01 Compared to Dedicated Diagnostic [99mTc]Tc-EDDA/HYNIC-iPSMA in Prostate Cancer
E Mamlins, L Scharbert, J Cardinale, M Krotov, E Winter, H Rathke, B Strodel, AO Ankrah, M Sathekge, U Haberkorn, C Kratochwil, FL Giesel
Molecular imaging and biology (2024)
Discovery of all-D-peptide inhibitors of SARS CoV 2 3C-like protease
R. Eberle, M. Sevenich, I. Gering, L. Scharbert, B. Strodel, K. Santur, J. Mohrlüder, M. Coronado, D. Willbold
ACS chemical biology (2022)
Novel Inhibitors of the Main Protease of SARS-CoV-2 Identified via a Molecular Dynamics Simulation-Guided in Vitro Assay
J. Loschwitz, A. Jäckering, M. Keutmann, M. Olagunju, R. J. Eberle, M. A. Coronado, O. O. Olubiyi, B. Strodel
Bioorg. Chem. 111, 104862 (2021)
High Throughput Virtual Screening to Discover Inhibitors of the Main Protease of the Coronavirus SARS-CoV-2
O. O. Olubiyi, M. Olagunju, M. Keutmann, J. Loschwitz, B. Strodel
Molecules 25, 3193 (2020)
This project is funded by VolkswagenStiftung.
Methods and software development
Hybrid force fields that combine all-atom and coarse-grained representations of the studied systems have been gaining more attention in the recent years to solve the time-scale problem in the molecular dynamics simulation. For instance, representing the protein using an all-atom force field while treating the solvent with a coarse-grained model could help reaching longer simulation times, with the cost of accuracy. We are working on developing such a force field for the amyloid-beta 42 protein to further understand its aggregation.
We develop software for the prediction of mutation effects on enzyme properties, including enantioselectivity, absoprtion, and fluorescence. To this end, we investigate the application of machine learning approaches, including transfer learning from large genome databases, to create precise predictions. Furthermore, we are maintaining Python codes to calculate and build transition networks for protein aggregation.
All the software and force fields developed by our group are available publicly on our GitHub page: https://github.com/strodel-group
TransMEP: Transfer learning on large protein language models to predict mutation effects of proteins from a small known dataset
T Hoffbauer, B Strodel
bioRxiv, 2024.01. 12.575432
ATRANET – Automated generation of transition networks for the structural characterization of intrinsically disordered proteins
M. Schäffler, M. Khaled, B. Strodel
Methods 206, 18-26, (2022)
This project is funded by Forschungszentrum Jülich.